|
|
|
SYSTEMATIC PALEONTOLOGY
Order RODENTIA Bowdich, 1821 1967 Peromyscus valensis Shotwell, p. 9; fig. 5 1977 ?Copemys vasquezi Jacobs, p. 512; Text-fig. 2 a-c 1979 Copemys sp. Baskin, p. 703 1985 Copemys near C. valensis Lindsay and Jacobs, p.19; fig. 8 a-b 1987 Copemys twenteri Czaplewski, p. 86; fig. 8 1994 ?Copemys Korth, p. 233 Type species. Postcopemys repenningi, new genus and new species Referred species. Postcopemys valensis (Shotwell 1967), Postcopemys vasquezi (Jacobs 1977), and Postcopemys maxumensis, new species. Distribution. Late Hemphillian and early Blancan of the North American Land Mammal Ages, including the Verde, Redington faunas, and possibly the White Cone fauna in Arizona; the Little Valley, McKay Reservoir, and Bartlett Mountain faunas in Oregon; and possibly the Yepómera fauna in Chihuahua. Diagnosis. Small, brachydont cricetid rodents having protolophule II not aligned with the anterior arm of the hypocone in upper molars, but with the entolophulid aligned with the anterior arm of the protoconid in lower molars. Protolophule I is absent in M1, it is usually present but weakly developed in M2 and is strongly developed in M3. The hypocone of M3 is reduced. Upper cheek teeth have three prominent roots and lack accessory rootlets; lower cheek teeth have two prominent roots and lack accessory rootlets. Discussion. In his emended diagnosis of the genus Copemys, Lindsay (1972:75) included the condition wherein the "metalophule on M1-2 [is] weakly developed, not aligned with anterior arm of hypocone," and in his differential diagnosis, he distinguished the genus Copemys from the genus Peromyscus in having "nonalignment of metalophule-anterior arm of hypocone [in upper molars] and entolophulid-posterior arm of protoconid" [in lower molars]. However, from his cusp terminology diagram (Lindsay 1972, figure 40), the protolophule II is clearly the name of the intended loph where the term "metalophule" was used in the text (see also Jacobs 1977, Baskin 1979). Therefore, Copemys is distinguished from its descendant, Peromyscus on the basis of not having alignment of protolophule II with the anterior arm of the hypocone in upper molars, and not having alignment of the entolophulid with the posterior arm of the protoconid in lower molars. In the genus Peromyscus these lophules and cusps are aligned, or nearly so. The genus Antecalomys Korth 1998 is somewhat intermediate with respect to these features in that lophs and cusps in upper molars are not aligned (as in Copemys), but lophs and cusps in lower molars are aligned as in Peromyscus. When Korth (1998) created this intermediate step by naming and describing Antecalomys he had in mind a transition between Abelmoschomys Baskin (1986) and Bensonomys Gazin (referred to Calomys Waterhouse by Baskin), placing Antecalomys between Abelmoschomys and Calomys (Bensonomys), in a lineage of putative sigmodontine rodents. Baskin (1986) had previously described Abelmoschomys, suggesting it as a likely ancestor of Calomys (Bensonomys) and other sigmodont rodents. One of the other prime features that Baskin (1986) noted in Abelmoschomys is the development of accessory rootlets, especially in the M1, noting that Abelmoschomys shares this feature with Sigmodon Say and Ord and with Calomys Waterhouse, as well as some other sigmodontines. Korth (1998) also noted presence of an accessory rootlet in M1 of the genotype of Antecalomys, A. phthanus, from the Pratt Quarry in Nebraska. Pardiñas et al. (2002) monographed our knowledge of the fossil and living South American and fossil North American Cricetidae including sigmodontines and presumed sigmodontines. These authors cogently argued that the North American fossils that are often referred to as Sigmodontinae have never actually been demonstrated monophyletic in a phylogenetic analysis that includes all of the extinct and extant South American genera. Numerous molecular phylogenetic analyses conducted in recent years show South American sigmodontines as monophyletic and separate from neotomine-peromyscine cricetids (e.g., Almeida et al. 2007; Jansa and Weksler 2004; Miller and Engstrom 2008; Reeder et al. 2006). On the other hand, there is no known ancestor in South America for the Sigmodontinae of South America, and the record of alleged Sigmodontinae in North America have a long and robust record that is older than any known record of a South American Sigmodontinae. We must admit there is a great deal of uncertainty regarding the evolutionary relationships among the New World cricetids (Musser and Carleton 2005:p. 1048; D'Elia 2000), and this uncertainty is likely to persist for a long time. We offer the present contribution as a tiny step toward resolution of that problem. In the mean time, it is prudent to consider the North American fossils as neotomine-peromyscines, or possibly sigmodontines, and we suggest use of the generic name Bensonomys Gazin for the North American taxon rather than Calomys (Bensonomys) Waterhouse, at least until more phylogenetic studies are completed. Korth (1998) also referred two species to Antecalomys: Copemys valensis, orginally described by Shotwell (1967) from Hemphillian faunas in Oregon, and ?Copemys vasquezi, described by Jacobs (1977) from the Hemphillian Redington fauna in Arizona. Korth based his referral on the the fact that in all this material the protolophule II is not aligned with the anterior arm of the hypocone in upper molars but the entolophulid is aligned with the posterior arm of the protoconid in lower molars. However, alignment of the entolophulid with the posterior arm of the protoconid that occurs in Copemys valensis and ?Copemys vasquezi may have little or nothing to do with the genus Antecalomys and the "sigmodontine" cricetids. As a general rule, accessory rootlets are never common in either Copemys Wood or Peromyscus Gloger. Also, unfortunately, roots and rootlets are rarely described for small rodents. To our knowledge, this feature, development of accessory rootlets is not commonly seen in any of the other Pliocene species of North American cricetid rodent although it developed in the late Miocene genus Abelmoschomys. Yet it separates the type species of Antecalomys, A. phthanus, from all the other Pliocene species of North American cricetid rodent that develop loph-cusp alignment in lower molars, such as Copemys valensis, ?Copemys vasquezi, and Copemys twenteri from the Verde fauna (Czaplewski 1987b). We assign these last three named species to the new genus Postcopemys along with the new species Postcopemys maxumensis from the Maxum fauna that we describe below. We suggest that the only species that should be assigned to Antecalomys is the type, A. phthanus, based on the presence of accessory rootlets on M1. Two other poorly known Hemphillian cricetids [Copemys sp. from the White Cone fauna, Arizona (Baskin 1979) and Copemys near C. valensis from the Yepómera fauna, Chihuahua (Lindsay and Jacobs 1985)] resemble Antecalomys and Postcopemys. They also tend to align the entolophulid with the posterior arm of the hypoconid in the lower molars although alignment (which is gradational) is less well developed, and upper molars of these taxa lack alignment of protolophule II with the anterior arm of the hypocone, as seen in Copemys. We discuss these taxa in comparisons of Postcopemys repenningi. We consider Postcopemys to be a member of the neotomine-peromyscine radiation of Cricetidae, along with Copemys and Peromyscus.
Postcopemys repenningi new genus and new species
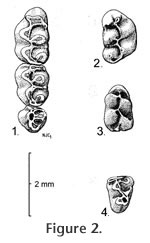
Holotype. MNA V4996, left M1-2 Type locality. MNA locality 701, Buckboard Wash, Verde Fm., Yavapai County, Arizona Hypodigm. Holotype plus MNA nos. V5003, associated right and left M1s and right m1; V5004, associated right and left M1s and right m1; V5000, left M1; V5001-5002, V5014, M1s; V4999, V5015-5016, V5022, M2s; V4997, V5008-5009, M3s; V5007, V5011-5013, m1s; V5017, right m2; V4998, V5006, V5010, m3s from MNA loc. 701. Material from other Verde sites: V5018, left M1; V5019-5020, left m1s from MNA loc. 698. V5005, left M2; V5021, left m1 from MNA loc. 319. Material from Maxum site: 35 isolated cheek teeth: UCMP 87472, 87473, 87477, 87478, 87481, 87483, 87487, 87496, 87519, 87525, 87535, 87537, 87542, 87543, 87545, 87552, 87557, 87558, 87559, 87561, 87565, 87897, 87899, 87901, 87905, 87921, 87924, 87925, 87926, 87928, 87943, 87950, 87958, 87963, and 87965 from UCMP locality V6869.
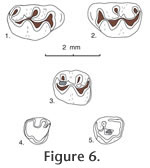
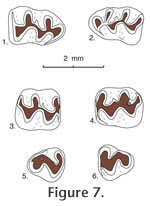
Age. Early Blancan and possibly latest Hemphillian NALMA. Etymology. Named for Charles A. Repenning, a weathered, spirited, decorated, kind, and always helpful gentleman paleontologist. Diagnosis. As in the generic diagnosis of Postcopemys above; also, P. repenningi differs from other species of Postcopemys in having smaller size than P. maxumensis and larger size than P. valensis and P. vasquezi. In addition, P. repenningi has a short- to medium-length mesoloph in M1 and M2; mesolophids are absent in m1 and m2. Description. The molars are low crowned and have thick enamel. Main cusp positions are slightly alternate with lingual cusps anteriorly situated relative to labial cusps.
M2. The anterior cingulum is reduced in its lingual portion. A weak protolophule I is present connecting the paracone with the anterior arm of the protocone. A strong protolophule II connects the paracone to the posterior arm of the protocone. Protolophule II is not aligned with the anterior arm of the hypocone. The mesoloph is of short- or medium length and does not reach the mesostyle. Three roots are present, and accessory rootlets are absent. M3. This tooth is small, and its occlusal outline is subcircular, wider than long. Strong protolophules I and II unite the protocone and paracone. The hypocone is reduced. m1. Occlusal outline is relatively long, narrow, and rounded anteriorly. Five distinct cusps are present but the metaconid is situated close to the anteroconid so that the intervening valley is quite shallow. The anteroconid is relatively short, broad, conical, and symmetrical in most specimens. In two of eight specimens the anteroconid is weakly bilobed. A strong anterior cingulum is present. Lophulids connecting cusps are weak. Mesolophid and mesostylid are absent. The entolophulid is aligned with the posterior arm of the protoconid. The posterior cingulum is shortened. Accessory rootlets are unknown. m2. Occlusal outline is longer than wide. Mesolophid and mesostylid are absent. The entolophulid is aligned with the posterior arm of the protoconid. The posterior cingulum is well developed. Accessory rootlets are unknown. m3. Occlusal outline is subtriangular, longer than wide. The hypoconid is reduced. The entoconid is indistinct. The posterior cingulum extends from the posterolingual side of the hypoconid to the posterior side of the entoconid, enclosing a deep pit. A broad short mesolophid(?) or lingual cingulum extends anterolingually from the entoconid.
P. repenningi is also similar in size to the unnamed species of Copemys from the White Cone l.f. of Arizona described by Baskin (1979) and the Copemys near C. valensis from Yepómera described by Lindsay and Jacobs (1985), both of which have protolophule II non-aligned with the anterior arm of the hypocone in upper teeth and alignment (more or less) of the entolophulid with the posterior arm of the protocone in lower molars. One M1 of Copemys near C. valensis has a slight bulbous expansion below the paracone, suggesting possible incipient development of an accessory rootlet as in Antecalomys phthanus. It is likely that the Copemys material from the White Cone and Yepómera faunas will be assigned to a species of Antecalomys or Postcopemys when those samples have been enlarged. Discussion. Before leaving this topic we should also point out that at least one extant species of the common North American genus Peromyscus, namely Peromyscus eremicus, should probably also be assigned to the genus Postcopemys based on dental morphology. An important study of dental variation in Peromyscus was published by Hooper (1957) in which variation in loph attachment and development of the mesoloph (id) and stylar cusps were reviewed and tabulated in 17 extant species of Peromyscus, including M1-2 and m1-2 of P. eremicus (Hooper 1957). Hooper's study stimulated EHL who in 1965 was struggling to identify fossil rodents collected from the Miocene Barstow Formation in southern California. Lindsay wanted to compare dental variation in modern species of Peromyscus—especially in large samples in which sex of the specimens had been recorded—in order to assess dental variation in fossil material that had been assigned by previous authors to Peromyscus. A prime goal was to see if some of the dental variation ascribed to Peromyscus by Hooper might be related to sexual dimorphism. Fortunately, large collections of Peromyscus species had been curated as skins and skulls in the Museum of Vertebrate Zoology at Berkeley, collected by Joseph Grinnell and his colleagues over several years, and sex of the preserved specimens had been recorded along with other important information. Some of the results of that study were summarized by Lindsay (1972, pp. 71-74) in his publication of the Barstow study. In summary, Lindsay found no dental variation that could be correlated with sexual dimorphism of the extant specimens, but after spending several days measuring and recording dental variation in extant Peromyscus, Lindsay realized that he could distinguish the dentition of extant Peromyscus from the dentition of fossils that had been assigned to Peromyscus. The difference was seen in the occlusal wear of the specimens. Extant Peromyscus species had developed alignment of protolophule II with the posterior arm of the hypocone to form an oblique ridge in upper teeth that would occlude against an oblique ridge (oriented at an angle of ~60-80 degrees) in lower molars formed by the entolophulid aligning with the posterior arm of the protoconid. None of the Miocene specimens assigned to Peromyscus exhibited that type of efficient grinding because the lophs and cusps were not properly aligned to enable such occlusion. Realizing that Miocene cricetids from Barstow can, and should be, distinguished from extant Peromyscus, Lindsay looked for another genus to which the Miocene cricetids could be assigned. Hoffmeister (1959) had described Miochomys niobrariensis from the Niobrara Formation in Nebraska, with the holotype located in the UCMP collection, readily available to Lindsay. So, Lindsay compared Miochomys niobrariensis with the cricetids from the Barstow Fm., finding them generically identical; when he completed his study of the Barstow small mammals Lindsay assigned all of the fossil cricetids to the genus Miochomys Hoffmeister. Later Lindsay presented the results of his Barstow small mammal study at an annual meeting of the Society of Vertebrate Paleontology held at Yale University, assigning the Barstow cricetid rodents to Miochomys Hoffmeister. Immediately after his presentation Repenning introduced Lindsay to V. Fahlbusch (in the restroom of the Peabody Museum), who had borrowed the type of Copemys loxodon, described by Wood (1936), wherein Fahlbusch informed Lindsay that features ascribed to Copemys loxodon were in error, and that most North American cricetids should be assigned to the genus Copemys Wood. Fahlbusch produced the type specimen of Copemys loxodon and upon examination Lindsay agreed with Fahlbusch's interpretation. Revisiting the study of dental variation in Peromyscus by Hooper (1957), Hooper illustrated M1-2 and m1-2 for 6 of the 17 species that he used: Peromyscus eremicus, figure 3; Peromyscus melanophrys, figure 9; Peromyscus oaxacensis (presently considered a synonym and tentative subspecies of P. aztecus; Musser and Carleton 2005, p. 1063), figure 15; Peromyscus mexicanus, figure 16; Peromyscus nuttalli (now = Ochrotomys nuttalli), figure 18; and Peromyscus nudipes (now considered a synonym of P. mexicanus; Musser and Carleton 2005, p. 1074), figure 19. In all of the lower teeth of his illustrated taxa the entolophulid is aligned with the posterior arm of the protoconid. In the upper teeth of some taxa (e.g., P. eremicus and P. oaxacensis [= P. aztecus]) protolophule II is not aligned with the anterior arm of the hypocone, and in some taxa (e.g., P. melanophrys and Ochrotomys nuttalli) alignment of protolophule II with the anterior arm of the hypocone is incomplete, reaching alignment only after moderate wear; in still other taxa (e.g., P. mexicanus including specimens formerly called P. nudipes) alignment of protolophule II with the anterior arm of the hypocone is complete with little or no wear. It is P. eremicus and P. oaxacensis (= P. aztecus) that show non-alignment of protolophule II with the anterior arm of the hypocone in M1 and M2; these are the features seen in Postcopemys, suggesting that at least these two species might be evolutionarily related and should probably be assigned to Postcopemys, as P. eremicus and P. aztecus. On the other hand, it would be inappropriate to reassign these species to another genus without a thorough review of all North American species of Peromyscus, which is beyond the scope of this study. We leave this task to interested students of evolution. When he examined the extant species of Peromyscus in the collection of the Museum of Vertebrate Zoology many years ago, Lindsay did not realize that Peromyscus eremicus lacked alignment of protolophule II with the anterior arm of the hypocone; he confined his study at the MVZ to samples with 20 males and 20 females of the same species, which did not include P. eremicus. Later, when Lindsay moved to southern Arizona where P. eremicus is the dominant cricetid rodent in the local fauna, he became acquainted with P. eremicus and realized the distinctiveness of its dental morphology relative to his previous interpretation. The present paper is the first time he reviewed and addressed its taxonomic relationships. In subsequent years much additional morphological and molecular genetic research on the systematics of extant cricetids has been accomplished. Ochrotomys nuttalli is widely considered a distinct monotypic genus and an early lineage of Neotominae separate from Peromyscus (Musser and Carleton 2005:p. 1061). Two subgenera of Peromyscus, P. (Peromyscus) and P. (Haplomylomys), which may or may not represent phyletic lineages, are presently recognized. Of these, P. (Haplomylomys) contains the P. eremicus and P. californicus groups of species, and P. (Peromyscus) contains all other extant species (Musser and Carleton 2005, p. 1062).
Postcopemys maxumensis, new species
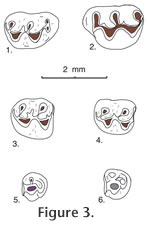
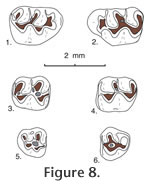
Type locality. UCMP locality V6869. Hypodigm. Holotype and 24 isolated cheek teeth: UCMP 87891, 87892, 87893, 87895, 87896, 87898, 87909, 87910, 87912, 87914, 87918, 87920, 87923, 87927, 87929, 87930, 87937, 87939, 87942, 87947, 87951, 87952, 87954, and 87973. Age. Early Blancan Etymology. ensis, for occurrence in the Maxum local fauna. Diagnosis. Larger size than P. repenningi and with a smaller bilobed anterocone on M1. Similar to P. repenningi in that the upper molars lack alignment of protolophule II with the anterior arm of the hypocone, and the lower molars have the entolophulid aligned (or nearly aligned when unworn) with the posterior arm of the protoconid; also similar to P. repenningi in having a short- to medium-length mesoloph and a minute mesolophid. Anterocone of M1 bilobed with lingual cusp slightly smaller than labial cusp. Anteroconid of m1 single-cusped and slightly asymmetrical.
M1. Occlusal outline is rounded oval, longer than wide; the anterocone is bilobed with the lingual lobe smaller than the labial lobe. Anterior arm of the protocone is long, joining the anterocone centrally near the midline; posterior arm of the protocone is short, joining protolophule II and the short anterior arm of the hypocone near the midline. Anterior arm of the hypocone is short, not aligned with protolophule II; metalophule II is directed lingually or posteriorly from the metacone to join the posterior cingulum labial to the midline. An anterior cingulum is low but persistent anterior to the anterocone; the lingual anterior cingulum descends from the lingual lobe of the anterocone, continuing to the base of the enamel. The anterior arm of the hypocone weakly reaches the labial side of the metacone in all specimens; metalophule II is well developed, directed posteriorly to join the posterior cingulum labial to the midline. M2. Occlusal outline is a rounded rectangle, longer than wide; anterior arm of the protocone is short, joining the anterior cingulum high on the midline; posterior arm of the protocone is long, joining protolophule II near the midline, and more posteriorly joining the anterior arm of the hypocone slightly labial to the midline; posterior arm of the hypocone joins metalophule II. A slight remnant of protolophule I is directed labially from the anterior side of the protocone, barely reaching the base of the paracone; protolophule II is prominent. The anterior cingulum is high and long labial to the midline, and separate from the paracone; it descends gradually lingual to the midline and is flexed posteriorly to terminate at the anterior base of the protocone. M3. Occlusal outline is well rounded, with two small cusps (protocone and paracone) plus a minute hypocone; the metacone is indistinct or absent. Protolophule I and II join the anterior and posterior sides, respectively, of the protocone, enclosing a small central basin. A mesoloph is indistinct or absent. The anterior cingulum is high labial to the midline, descending rapidly lingual to the midline; the posterior cingulum is directed labially from the minute hypocone, terminating at the posterolabial corner of the tooth; a labial cingulum is low or indistinct. m1. Occlusal outline is subrectangular, longer than wide, narrow and rounded anteriorly. The anteroconid is single cusped, relatively narrow and slightly asymmetrical. Short anterior arm of the protoconid joins the metalophulid and short anterolophid centrally; posterior arm of the protoconid is relatively long, joining a labially-directed entolophulid and more posteriorly joining the anterior arm of the hypoconid labial to the midline; entolophulid is short, weakly joining the posterior side of the metaconid in 2 of 4 specimens. Anterior arm of the hypoconid broadly joins the entolophulid on the midline; posterior arm of the hypoconid is directed labially, becoming the posterior cingulum. The anterior cingulum is high on the labial side of the anteroconid, descending posteriorly; on the lingual side of the anteroconid the anterior cingulum is indistinct in 3 specimens, short in 2 specimens; a posterior cingulum is directed lingually, and slightly expanded posteriorly in 2 of 3 specimens; a low labial cingulum partially closes the labial sinus. m2. Occlusal outline is a rounded rectangle, longer than wide. Short anterior arm of the protoconid joins metalophulid I and the anterior cingulum high on the midline; long posterior arm of the protoconid joins the short anterior arm of the hypoconid and entolophulid lingual to the midline; long posterior arm of the hypoconid is flexed lingually and slightly expanded posteriorly as the posterior cingulum. A minute mesolophid is directed lingually from the posterior arm of the protoconid in 2 of 5 specimens, terminating short of the metaconid; the entolophulid is short, directed posteriorly from the entoconid and weakly joining the posterior side of the hypoconid in 2 of 4 specimens. The anterior cingulum is high on the midline, gradually descending labially, and flexed posteriorly to terminate at the anterior base of the protoconid; the anterior cingulum is absent or indistinct lingual to the midline; a low cingulum partially closes the labial sinus. m3. Occlusal outline is a rounded triangle, narrow posteriorly. Three cusps (protoconid, small metaconid, and smaller hypoconid) are distinct, and the entoconid is indistinct or absent. The short anterior arm of the protoconid and metalophulid I join near the anterior tooth margin lingual to the midline; the long posterior arm of the protoconid joins the anterior arm of the hypoconid lingual to the midline, and apparently continues lingually (? as a mesolophid) to terminate short of the lingual tooth margin. The metaconid and hypoconid are too worn to show significant features. All of the specimens are well worn, and the anterior cingulum is preserved only labial to the midline where it is restricted to the anterolabial corner of the tooth. A lingual cingulum is high in the position of the entoconid, and continues posteriorly to merge with the posterior cingulum and the hypoconid; anterior to the position of the entoconid the lingual cingulum descends slightly, to close a deep, narrow basin posterior to the metaconid. Comparisons and DiscussionPostcopemys maxumensis new species, is larger than known species of Postcopemys, e.g., P. repenningi, P. valensis, and P. vasquezi; in addition, the anterocone on M1 of P. maxumensis is more elongate, with a low anterior cingulum. The m1 anteroconid is single cusped and slightly asymmetrical; the M3 is relatively large, with a minute hypocone; and m3 is relatively short and wide, compared to P. repenningi, its closest analogue. P. maxumensis differs from Antecalomys phthanus in lacking accessory rootlets. P. maxumensis is assigned to Postcopemys because the upper molars lack alignment of protolophule II with the anterior arm of the hypocone, and the lower molars have the entolophulid aligned (or nearly aligned when unworn) with the posterior arm of the protoconid.
Jacobsomys dailyi
May, Woodburne, Lindsay, Albright, and Sarna-Wojcicki,
2011
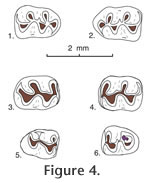
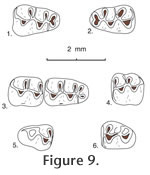
Localities. UCMP localities RV-7702, Warren local fauna; RV-8103, upper Horned Toad Hills local fauna; and V-6869, Maxum local fauna, all in California. Age. Late Hemphillian and early Blancan. Description. Jacobsomys dailyi is a medium-sized, brachydont cricetid rodent with a large bilobed anterocone on M1 and a large bilobed anteroconid on m1. In addition, labial cusps are placed opposite the posterior side of lingual cusps in upper molars, and lingual cusps are placed opposite the anterior side of labial cusps in lower molars; a long, thin mesoloph and mesolophid are usually present in upper and lower molars, and a thin, labial anteroloph is often present in M1, along with a long, thin, labial anterolophid in m1. These lophs are always situated anteriorly, tending to merge with the anterior lingual cusp in upper molars; in lower molars the anterolophid is situated anteriorly, tending to merge with the labial lobe of the anteroconid, whereas the mesolophid is situated posteriorly, tending to merge with the lingual entoconid; all of these lophs and lophids tending to fuse (with wear) to the base of the adjacent main cusp. Protolophule II, metalophule II, and entolophulid tend to bifurcate toward their base with the medial branch joining the mure near the midline, and the lateral branch joining the loph or lophid directed away from the mure. Thus, these teeth tend to develop three lophate transverse ridges in M1 (anteroloph, mesoloph, and posterior cingulum) and in m1 (anterolophid, mesolophid and posterior cingulum [with the metaconid of m2]). There are only two lophate ridges on M2 and m2. The enamel is worn quickly near the base of these ridges, especially at the posterior base of labial cusps in M1-2 and the anterior base of lingual cusps in m1-2, presumably the result of a strong power stroke in mastication. Also, a posterolabial sulcus is developed between the robust posterior cingulum and the hypoconid in m1 and m2. Roots, where known, are well developed, lacking accessory rootlets; upper molars tend to elongate the lingual root anteroposteriorly, extending beneath the hypocone in M1; lower roots tend to develop a thin bony wall medially (in m1), or increase the width of the posterior root (in m2). The dentary has a broad, prominent masseteric crest whose anterior termination is blunt, below the anterior root of m1; the masseteric crest swells posterior to its anterior termination, becoming more ridge-like. The mental foramen is exposed slightly anterior to the masseteric crest, on the posterolateral side of the diastema, slightly below the level of the anterior termination of the masseteric crest.
M2. Occlusal outline is subrectangular, longer than wide, with a straight and steep anterior side. Protolophule I is distinct in 4 of 5 specimens, joining the anterior arm of the protocone (3 specimens), or the labial side of the anterior cingulum (1 specimen); protolophule II is present in 4 of 5 specimens, bifurcating in 3 specimens with the anterior branch joining the posterior arm of the protocone and the posterior branch (if present) joining the middle of the mesoloph; metalophule II is present in 5 specimens, bifurcating in 4 of those with the lingual branch joining the posterior arm of the hypocone where it joins the posterior cingulum and the labial branch weakly joining the labial end of the posterior cingulum. The anterior cingulum is high labially, separate from the paracone, and descends rapidly lingual to the midline. M3. Occlusal outline is subcircular, with a straight anterior side. The protocone and paracone are well developed although small relative to cusps on anterior teeth; the hypocone is small and the metacone is indistinct (9 specimens) as a swelling on the labial cingulum or minute (2 specimens). The anterior cingulum is long and prominent, joining the anterior arm of the protocone and the anterolabial paracone; a high, narrow loph (?protolophule I) is present in 9 of 11 specimens, joining the anterior arm of the protocone (5 specimens) or the anterior cingulum (4 specimens); another loph (?protolophule II) is directed posterolingually from the paracone, joining the anterior side of the hypocone to enclose a deep, central basin; another short loph is directed labially from the hypocone toward the posterior cingulum, terminating at a swelling (?metacone) on the cingulum, to enclose (with the posterior cingulum and ?protolophule II) a shallow posterior basin. m1. Occlusal outline is an elongated rectangle, narrow anteriorly. The long anterior arm of the protoconid joins the short metalophid I on the midline and continues anteriorly to join centrally the lobes of the anteroconid; a low and thin anterolophid is usually directed labially (8 of 9 specimens) from the junction of the arm of the protoconid with the anteroconid, terminating at the labial tooth margin; the long posterior arm of the protoconid joins the base of the long, thin mesolophid and the short entolophulid near the midline. The short anterior arm of the hypoconid joins the posterior base of the entolophulid; posterior arm of the hypoconid is directed posteriorly and gently flexed lingually to continue as a prominent posterior cingulum that terminates short of the lingual tooth margin. Metalophulid I is bifurcated in only 2 of 9 specimens; entolophulid is bifurcated in 7 of 9 specimens, with the posterior branch joining the protoconid on the midline and the anterior branch joining the middle of the mesolophid. There is no distinct anterior cingulum descending from the lobes of the anteroconid, although low cingula partially close anterior sinuses on the labial and lingual sides of teeth. m2. Occlusal outline is subrectangular, longer than wide. The short anterior arm of the protoconid joins the short metalophulid I and continues to join the anterior cingulum high on the midline; the long posterior arm of the protoconid joins the base of the mesolophid and continues posteriorly to join the entolophulid. The short anterior arm of the hypoconid joins the posterior base of the entolophulid near the midline; the long posterior arm of the hypoconid is directed posteriorly and flexed lingually to continue as a prominent posterior cingulum that descends and terminates at the posterior base of the entoconid. Metalophulid I is bifurcated in 3 of 8 specimens; entolophulid is bifurcated in all 8 specimens. The anterior cingulum is close to (and obscured by) the metaconid lingual to the midline, it descends labially and is gently flexed posteriorly to terminate at the base of the protoconid; low labial cingula partly close the labial sinus, the lingual sinus is partly closed by the mesostylid, if present. m3. Occlusal outline is elongate oval, longer than wide and narrow posteriorly; three cusps are prominent with the protoconid and metaconid larger and slightly higher than the hypoconid; an entoconid is indistinct or absent. The short anterior arm of the protoconid joins a short metalophid I and continues anteriorly to join the anterior cingulum high near the midline; the long posterior arm of the protoconid joins the anterior arm of the hypoconid (with a smooth flexure) lingual to the midline, anterior to a broad juncture with a short loph (?entolophulid or mesolophid). The robust posterior arm of the hypoconid is directed lingually and continues around the posterior margin of the tooth as a narrow lingual cingulum that descends anteriorly to join a short loph (?entolophid or mesolophid). An anterior cingulum is high at the midline, merging with the base of the metaconid lingual to the midline, and descending labially very steeply to terminate short of the labial tooth margin; the labial sinus is partially closed by a low labial cingulum. Discussion. Jacobsomys dailyi is significantly smaller than Jacobsomys verdensis, the only other known species of this genus. When Czaplewski (1987a) described Jacobsomys verdensis he compared it to the living rice rat Oryzomys. Examination of modern rice rats indicates that they share many dental features with Jacobsomys; these two genera differ dentally primarily in that the m1 of Jacobsomys has a well-developed bilobed anteroconid, and the m1 of Oryzomys has a prominent single-cusped anteroconid. Both genera appear to share development of the anteroloph (and anterolophid) plus the mesoloph (and mesolophid) with bifurcating protolophules and metalophules in M1-2 as well as having a bifurcating entolophulid in m1-2, along with the wear pattern described above to characterize Jacobsomys. In addition, they share a long, thin anteroloph in M1, a long, thin mesoloph that bonds to the posterior side of the paracone in M1 and M2; a long, thin anterolophid in m1, and a long, thin mesolophid that bonds to the anterior side of the entoconid in m1-2. Jacobsomys and Oryzomys have developed a distinctive mastication reflecting unusual wear on the side of cusps on both upper and lower teeth that occlude with one another. For example, Oryzomys fulvescens develop, in upper molars, prominent sloping well-honed wear facets on the posterior side of labial cusps resulting from bifurcation of the protolophule and to some extent the metalophule; in lower molars they develop prominent, sloping, well-honed wear facets on the anterior side of lingual cusps, resulting from bifurcation or possible bifurcation of the metalophulid and entolophulid. These same features appear on Oryzomys palustris, in addition to lateral expansion of the anteroloph, mesoloph and posterior cingulum in upper teeth to enclose and expand a well-worn grinding surface. Occlusal wear on the side of prominent cusps seems characteristic of Oryzomys and Jacobsomys but wear also occurs at the tip of cusps. These rodents have developed a unique and efficient method of grinding favorite foods that initially appeared in Jacobsomys. Several fossil records have been assigned to Oryzomys in the literature. These include ?Oryzomys pliocaenicus from the Hemphillian Edson Quarry fauna, Kansas (Hibbard, 1937), ?Oryzomys from the Hemphillian Bartlett Mountain fauna in Oregon (Shotwell 1970), and cf. Oryzomys from the Truth or Consequences fauna Mexico (Repenning and May 1986); it now appears that these records should be checked for possible assignment to Jacobsomys rather than Oryzomys or ?Oryzomys. In summary, there is a strong similarity between Jacobsomys and Oryzomys, suggesting a possible ancestral-descendant relationship between these genera. |
|